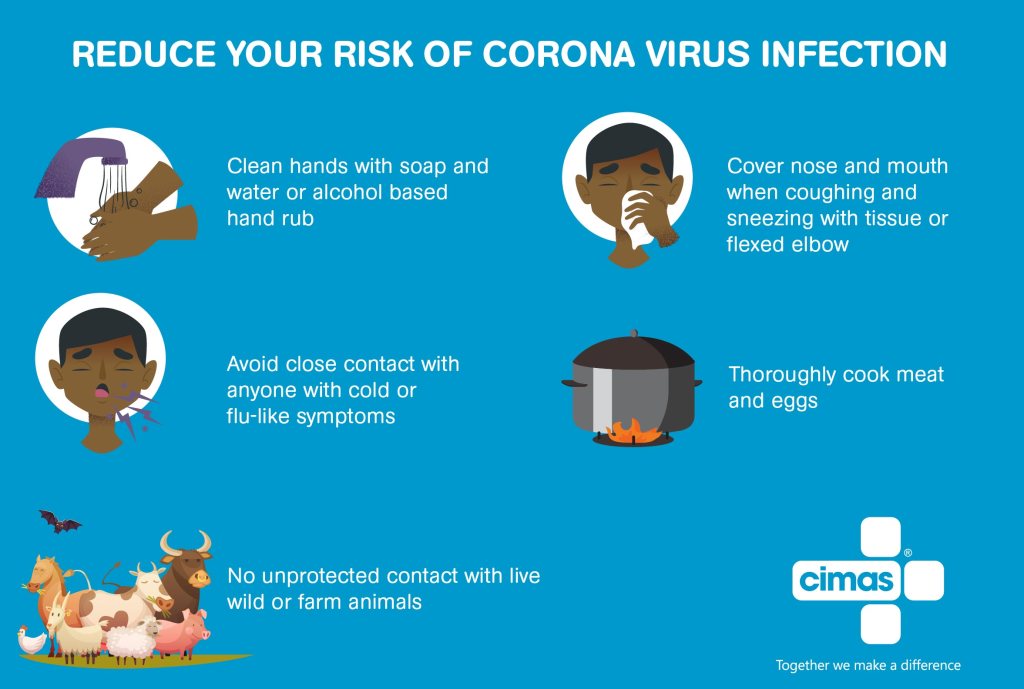
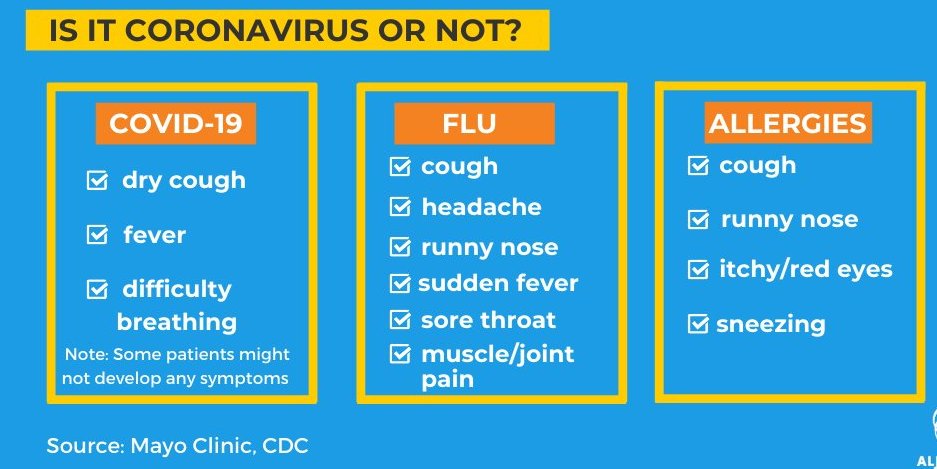
The
amorphous structures by the high degree of short range order and absence of long range order. From the energetic point of view,
atoms in an
amorphous crystal are not bonded ideally; they are subject to important stresses and distortions. The energy of an amorphous solid is thus higher than that of a
pure crystal. There are two specific amorphous form of carbon: the diamond-like amorphous carbon and the graphite-like amorphous carbon. These two structures can be distinguished clearly by their
macroscopic and
microscopic properties. The former has higher density, is transparent and much harder than the latter. From the microscopic point of view, the ratio of fourfold, diamond like bonds to threefold, graphite-like bonds will determine the kind of structure we obtain.